Lipopeptides
A lipid molecule is added to a peptide sequence during lipidation, a peptide modification process. Since lipopeptides can be used instead of potentially harmful adjuvants while still eliciting a thorough immune response, they are particularly interesting for vaccine development. Because synthetic peptides by themselves are insufficient to elicit an immune response, immunoadjuvants are frequently used to strengthen the reaction. In the development of peptide vaccines, alternatives to the traditional Freund’s adjuvants have met with some success. These include conjugations to viral and bacterial carrier proteins, immunostimulating complexes, and branching multiple antigenic peptides (MAPS).
After Hopp and colleagues discovered in the early 1980s that a peptide conjugated to a dipalmityl-lysine moiety increased anti-hepatitis surface antigen response, lipopeptides were initially used in vaccine development.[1] This result is explained by the fact that, despite the possibility of short peptides containing cytotoxic T cells (CTL) that bind to MHC class I molecules, they are unable to prime CD8+ T-cell responses in vivo. This was further supported by Rammensee and colleagues, who demonstrated in the early 1990s that an influenza lipopeptide (without the need for an adjuvant) evoked CTL responses but the comparable peptide without lipid conjugation did not.
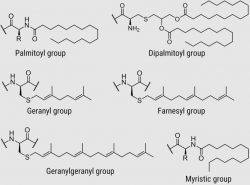
Myristic acid, palmitoyl, dipalmitoyl, geranyl, farnesyl, geranylgeranyl, and other lipids are only a few of the lipids that may be linked to nearly any peptide sequence by CPC due to its significant experience and knowledge in the synthesis of peptides containing lipid moieties.
Common Lipid Abbreviations
ABBREV.
FULL NAME
IUPAC NAME
PAL
Palmitic acid
Hexadecanoic acid
MYR
Myristic acid
Tetradecanoic acid
GER
Geranyl group
(2E)-3,7-dimethylocta-2,6-dien-1-yl
FAR
Farnesyl group
(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl
GGER
Geranylgeranyl group
(2E,4E,6E)-3,5,7,11-Tetramethyldodeca-2,4,6,10-tetraene-1-yl
References
Hopp, Thomas P. “Immunogenicity of a synthetic HBsAg peptide: enhancement by conjugation to a fatty acid carrier.” Molecular Immunology 21, no. 1 (1984): 13-16.