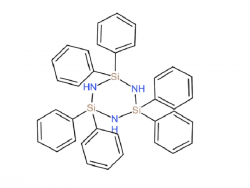
Chlorosilane
Chlorosilanes are a class of liquids that range in color from clear colorless to light yellow colored. A clear to light yellow liquid. Slightly more dense than water. Contact may burn skin, eyes and mucous membranes. May be toxic by ingestion, inhalation, and skin absorption. Used to make other chemicals.
Zhongtian Chlorosilane Product Series
As a local China silicone products manufacturer, the chlorosilane product series oofered by Zhongtian consists of methyltrichlorosilane, dimethyldichlorosilane and trimethyl chloro silane. Prepared by the reaction of silicon powder and methyl chloride (CH3CI) gas through direct synthesis, the methyl chlorosilanes produced from chlorosilane (trimethyl, dimethyl and methyl chlorosilane) are the components of various silicone substances. Chlorosilanes are used as silicon sources in the production of silicon epitaxial wafers and as raw materials for the preparation of organochlorosilanes
Methyltrichlorosilane (M1)
Methyltrichlorosilane is a colorless or yellow transparent liquid, which is the main material of silicone products.
Dimethyldichlorosilane(M2)
This product is a colorless transparent liquid, inflammable, explosive. Dimethyldichlorosilane is the raw material of silicone products, the reaction generated under the influence of water HCL, avoid sharp, contact heat, fire, and static electric spark.
Trimethylchlorosilane (M3)
Trimethylchlorosilane is a colorless or light yellow transparent irritating liquid.
Chlorosilane Synthesis
Chlorosilanes are prepared by reacting silica powder (derived from natural silica by an electrochemical process) with CH3CI or chloromethane gas (formed in a reaction between HCI and CH30H or methanol). This process is called the direct synthesis of chlorosilane, which is carried out in a large reactor and produces a crude mixture of several liquid chlorosilanes that must then be separated by distillation. The chlorosilanes then generated are the building blocks for the subsequent production of various silicone substances: the central silicon atom is combined with various numbers of chlorine atoms, each providing a potential reaction site for the hydrolysis reaction, allowing the length and branching of the polymer molecular backbone to be controlled. The three types of methylchlorosilanes obtained for silicone production during the direct synthesis are trimethyl chlorosilane, dimethylchlorosilane, and methyltrichlorosilane.
Chlorosilane Reactions
Chlorosilane is a chemical with the chemical formula SiH3Cl. It is a clear, water-like, flowable liquid. It is flammable but volatile. Easily hydrolyzed and fumes in the air. Quite inert to metals (when absolutely dry), chlorosilane does not even react with sodium metal. Produced by reaction of SiCl4 with hydrogen or SiH4 with chlorine. Chlorosilanes can react vigorously with compounds containing active hydrogen, such as water, alcohols, phenols, silanols, organic acids, etc., releasing hydrogen chloride. Chlorosilane reactions with organometallic compounds, the chlorine atom is replaced by the corresponding organic group to produce organic chlorosilane or organosilane. It is used as a silicon source in the production of silicon epitaxial wafers and as a raw material for the preparation of organic chlorosilanes.
Chlorosilane reactions vigorously with compounds containing active hydrogen, such as with water, alcohols, phenols, silanols, organic acids, etc., releasing hydrogen chloride.
Chlorosilane reactions with organometallic compounds, the chlorine atom is replaced by the corresponding organic group to produce organic chlorosilane or organosilane.
Chlorosilane Leakage Treatment
Evacuate people in the contaminated area of the spill to a safe area, prohibit extraneous personnel from entering the contaminated area, and advise emergency responders to wear self-contained breathing apparatus and chemical protective clothing. Do not touch the spill directly, do not let the spill come into contact with combustible materials (wood, paper, oil, etc.), and plug the leak in a safe manner. Spray water mist to slow volatilization (or diffusion), but do not spray water directly on the spill or leak site. Sprinkle the ground with soda ash, then flush with plenty of water and put the diluted wash water into the wastewater system. If a large leakage occurs, it is best to treat chlorosilane without water and remove it under the direction of a technician.
Health Hazards of Chlorosilane
Inhalation and ingestion have a strong irritating effect on the mucous membrane of the eyes and respiratory tract. At high concentration, chlorosilane can cause corneal clouding, respiratory inflammation, and even pulmonary edema, and can be accompanied by dizziness, headache, weakness, nausea, vomiting, panic, and other symptoms. Splashing on the skin can cause necrosis and ulcers that do not heal for a long time.
Fire-fighting Measures of Chlorosilane
Extinguishing agents: Use water mist, dry powder, foam, or carbon dioxide extinguishing agents to extinguish the fire. Avoid using direct running water to extinguish the fire; direct running water may cause splashing of flammable liquids and spread the fire.
Firefighting precautions and protective measures: Firefighters must wear air-carrying breathing apparatus and full-body firefighting suits and extinguish the fire upwind. Move the container from the fire to the open area as far as possible. If the container in the fire scene has changed color or made a sound from the safety relief device, it must be evacuated immediately. Isolate the accident scene and prohibit the entry of unrelated personnel. Take in and dispose of fire water to prevent pollution of the environment.
Description of Silicone Monomer
Silicone monomer is the ingredients for materials processing of silicone oil, silicone rubber, silicone resin, and silane coupling agent. Thousands of silicone products can be produced from several basic silicone monomers. The classification of silicone monomer includes methyl chlorosilane (referred to as methyl monomer), phenylchlorosilane (referred to as phenyl monomer), methylvinylchlorosilane, ethyl trichlorosilane, propyltrichlorosilane, vinyl trichlorosilane, γ-chloropropyltrichlorosilane and fluoro silicone monomer, etc. Among them, methyl chlorosilane is the most important, and its amount accounts for more than 90% of the total monomer, followed by phenylchlorosilane.
Usage of Silicone Monomer
The key to the development of any polymer material lies in the development of monomer technology. The silicone industry is characterized by concentrated silicone monomer production and decentralized product processing. Therefore, silicone monomer production plays an important role in the organic silicon industry, and the production level of monomer directly reflects the development level of the organic silicon industry. Because silicone has these excellent properties, it has a very wide range of applications. It is not only used as a special material for aviation, cutting-edge technology, and military technology sectors, but also for various sectors of the national economy, and silicone monomer’s applications have been expanded to include: construction, electronics, and electricity, textiles, automobiles, machinery, leather and paper, chemical and light industry, metals and paints, medicine and medical treatment, etc.
The monomer of Silicone Industry
Silicones, known as “industrial monosodium glutamate,” are compounds that contain a silicon-carbon bond (Si-C) and have at least one organic group bonded to a silicon atom through the Si-C bond. Among them, polysiloxane, which is composed of a silicon-oxygen bond (-Si-O-Si-) skeleton, has the highest percentage (more than 90%) and is the most studied and widely used, because monomer of silicone has unique properties unmatched by other polymer materials, such as high and low-temperature resistance, moisture resistance, insulation, corrosion resistance, aging resistance, and physiological inertia.
The monomer of the silicone industry chain is mainly divided into four segments: raw materials, monomer, intermediates, and deep processing, of which monomer and intermediates are the core of the monomer of the silicone industry chain. The organosilicon industry is characterized by centralized monomer production and decentralized product processing. The key to the development of any polymer material lies in the development of monomer technology. Since the monomer of the silicone process is complex. The process is long, and the technical barriers are high, which is a capital-intensive and technology-intensive industry. In terms of downstream products, there are four major categories of silicone rubber (room temperature vulcanization rubber and high-temperature vulcanization rubber), silicone oil, silicone resin, silicone coupling agent, etc. There are more than 7,000 kinds of specific products, and the end-use applications are all over the construction materials, electronic appliances, medicine and health, chemical industry, automobile, textile and other industries.